The uga biopharma gmbh high speed workflow was established as an improved cell line development workflow that guarantees cho cell line development on a shortened time scale.
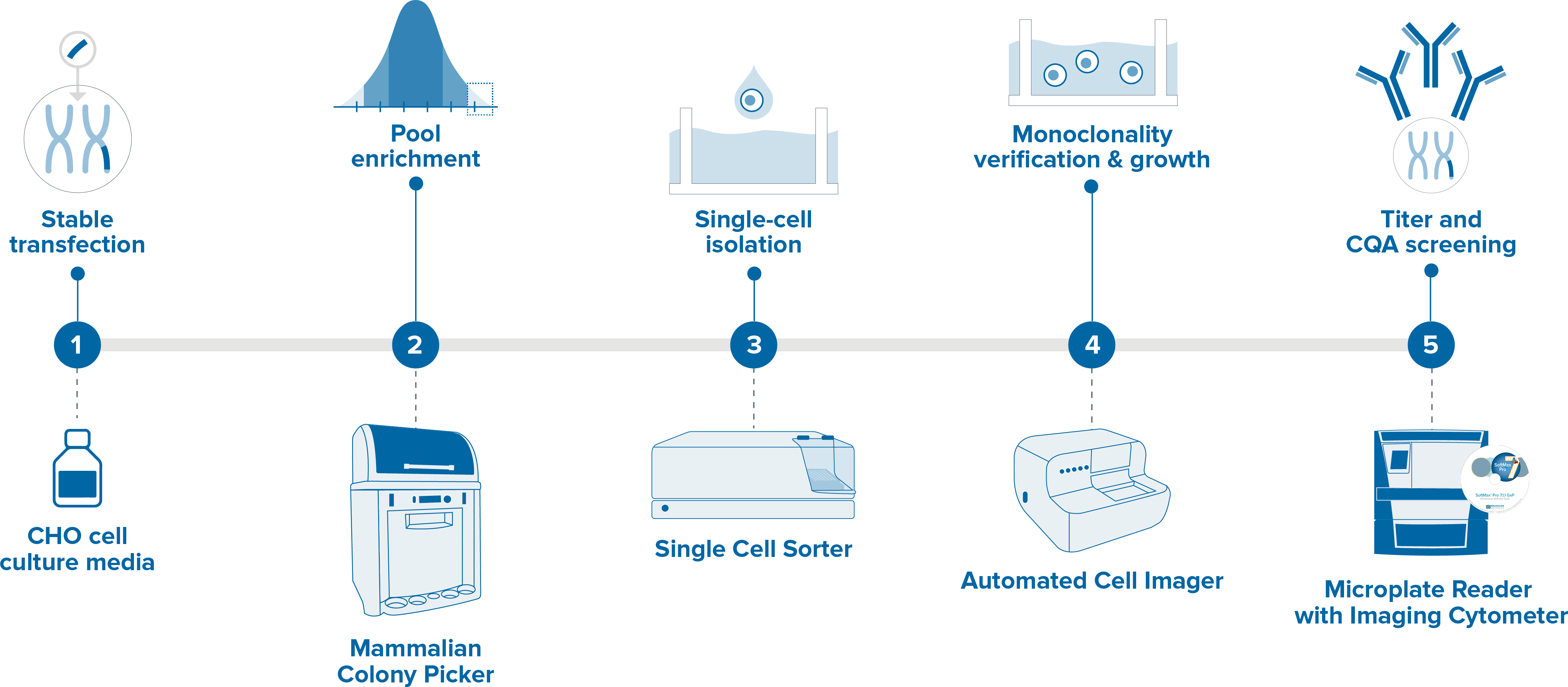
Cell line development workflow.
In the case of monoclonal antibody production the process of developing stable cell lines starts with transfecting host cells with recombinant plasmid dna encoding the.
Cell line development workflow in order to generate high yields of recombinant protein products stable cell lines such as cho or hek 293 are typical vehicles of choice.
Based on the suretechnology platform and unique world class expertise selexis sure cell line development services significantly reduce the time effort and costs that are associated with the development of high performance mammalian cell lines for therapeutic protein production e g development of monoclonal antibodies bi specific monoclonal antibodies growth factors and enzymes.
Cell line development campaigns are typically performed over the course of several weeks to months at a time.
Interested in rapid and efficient stable cell pool cell line development for large scale production of your recombinant antibodies and proteins.
Typical cell line workflow.
Cell line development cld on the beacon optofluidic system enables selection of the best production cell lines with 99 monoclonality assurance in just days.
Cell line development is the process of establishing a clonally derived cell population which has been genetically engineered to express a desirable phenotype such as producing large amounts of recombinant protein for a stable period of time.
The opto cld workflow screens thousands of clones with ease in less than a week.
The new opto cld 2 0 workflow extends these capabilities to allow 1 enrichment of rare cell populations 2 on chip titer measurements for traditional and non traditional antibody molecules and 3 efficient recovery.
The biomek software suite ensures data integrity to provide peace of mind and schedules activities to streamline each automated workflow.
From vector design and host cell selection prior to transfection single cell cloning and colony outgrowth right through to the establishment of the master cell bank solentim technologies provide the evidence for.
We accelerate cell line workflow timelines from upstream development through scale up to production of new biological therapies.
Solentim technology streamlines workflows in the creation isolation and characterization of high value cells in cell line development vaccine production cell and gene therapy.
For the greatest genetic diversity you need to screen thousands of cells.